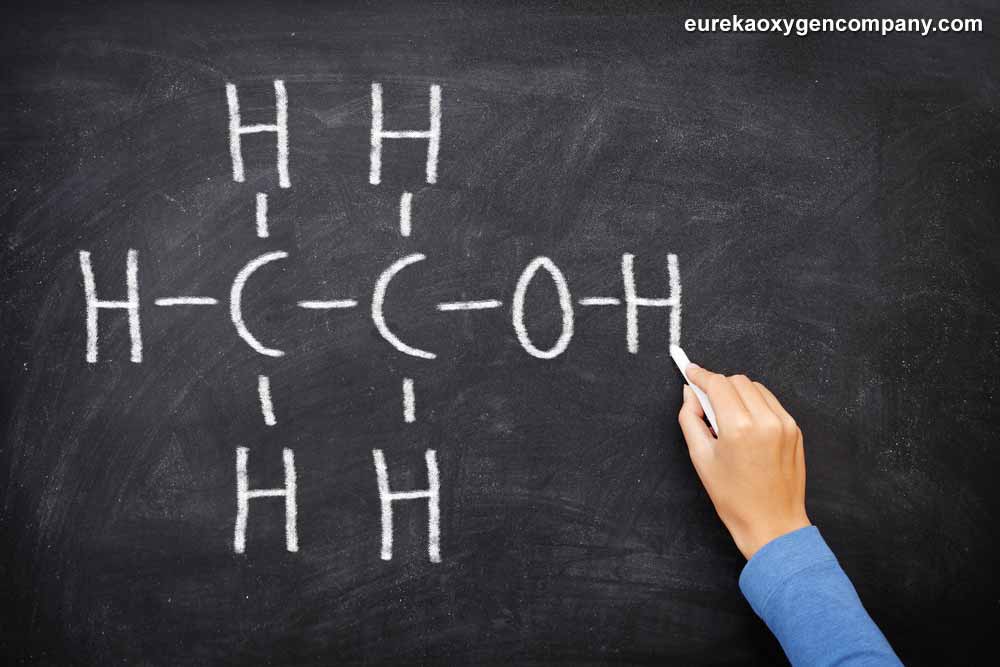
Ethanol, also known as grain alcohol, or even ethyl alcohol is an organic compound, which belongs to a class referred to as alcohols. The chemical formula for ethanol is C2H5OH. Generally speaking, this organic compound has a wide variety of applications. As a very important industrial chemical, ethanol is often utilized as a solvent. It is also used very commonly in the synthesis of many other organic chemicals. It is as well made use of as a key additive in the production of a range of gasoline that powers vehicle engines.
On the other hand, ethanol is utilized as an ingredient in diverse alcoholic drinks including beer, wine, and even distilled spirits. Ethanol is also used to produce a variety of household products such as perfumes, colognes, liniments, aftershaves, rubbing alcohol, mouthwashes to mention just a few. When it is meant to be used industrially, this organic compound normally undergoes a process known as denaturation, which is simply rendering it unfit for drinking. In such applications, either methanol, kerosene, or benzene are utilized to denature ethanol.
Ethanol as a fuel


Ethanol happens to be a renewable source of fuel that is produced from a number of different plant materials that are collectively called biomass. To this end, over 98% of gasoline produced in the United States contains some form of ethanol that is known as E-10 and consists of 90% gasoline and 10% ethanol. In this particular application, this organic compound is used to oxygenate the gasolene which goes a long way in minimizing air pollution. There is also a variant of ethanol referred to as E-85 that is specifically designed for flexible fuel automobiles.
For those who might be in the dark, flexible fuel vehicles are those which are made to utilize any mix of gasoline and ethanol, not more than 83%.
Another sort of ethanol fuel is E-15 which is designed for vehicles manufactured from the model year 2001 along with much newer light-duty automobiles. On the flip side, this organic compound reforming is currently being widely studied as a promising process for producing clean hydrogen fuel. This is simply because ethanol is produced using renewable feedstock and also possesses a high content of hydrogen. If successful, it is thought that ethanol reforming may produce high yields of hydrogen with total ethanol conversion at low temperatures.
Ethanol fuel properties
Ethanol is an essentially colorless and clear liquid. What makes it stand apart from other organic compounds is the fact that it possesses the same chemical formula despite the exact materials that are utilized to produce it. This is always the case whether sugar-based feedstock, sugarcane or cellulosic feedstock are used. Also, when compared with gasoline, ethanol possesses a much higher octane number. This provides it with much better (premium) blending properties. Reduced octane gasolene is usually prepared with 10% ethanol to reach the standard 87 octane.
Ethanol energy balance

Then it comes to the US, no less than 94% of ethanol is made with the starch found in corn grain, and some energy is necessary to convert raw feedstock into ethanol. The version of this organic compound that is made with corn has demonstrated a positive energy balance. In much simpler terms, this just means that producing ethanol gasoline doesn’t necessitate more energy than the amount of energy that is already in the gasoline itself. When compared to other variants of this organic compound, cellulosic ethanol is noted for improving the energy balance of gasoline.
This is simply because the feedstocks used to produce it are derived from waste, co-products of other industries (such as wood and crop residues) or even crops specifically grown to produce ethanol. The last of which may include switchgrass and miscanthus that are acclaimed for necessitating lower amounts of water and fertilizer when compared to corn. When biomass is picked to produce the energy needed to power the process which transforms non-food-based feedstocks into cellulosic ethanol, the amount of fossil fuel energy that is utilized in the production can be minimized even further. Yet another advantage of using cellulosic ethanol is its production often culminates in the emission of lesser amounts of life cycle greenhouse gases.
Ethanol as an ingredient of alcoholic drinks

Ethanol is usually categorized as a central nervous system depressant. While it possesses little medicinal value, ethanol is a key ingredient in the production of alcoholic drinks that are used for recreational purposes. Ethanol intake in this particular context is measured in terms of units. With a single unit being equal to 8 g of it which is the amount contained in ½ a pint of beer, one standard glass of wine, and one measure of a distilled spirit.
Eureka Oxygen is a foremost vendor of industrial fuels including ethanol and safety gear like fire suppression equipment. Besides this, it offers diverse welding products as well as welding equipment, welding supplies, and numerous welding accessories.